A team of researchers at the University of Macau (UM) has developed an innovative technology that combines nanoparticles, bacterial membranes, and immune cells to deliver drugs directly to tumours to attack cancerous cells. Led by Wang Ruibing, a supramolecular chemist and associate professor at UM’s Institute of Chinese Medical Sciences (ICMS) and State Key Laboratory of Quality Research in Chinese Medicine, the team seeks to bring about more effective and personalised cancer treatments at a lower cost.
Supramolecular Smart Biomaterials Improve Targeted Drug Delivery
Since joining UM as a faculty member in 2014, Prof Wang has brought a wealth of experience in supramolecular chemistry, supramolecular biomaterials, radiopharmaceuticals, and molecular bioimaging to the university. Elected a fellow of the Royal Society of Chemistry in 2020, Prof Wang studies how molecules interact with each other and assemble themselves through non-covalent bonding, which creates simple yet smart structures with unique biological properties. He describes these structures, known as supramolecular molecules, as ‘molecules holding hands’. Through studying supramolecular chemistry, scientists have developed molecular structures for various needs, such as medicines and healthcare products.
Hunting Down Solid Tumour Tissue
According to Prof Wang, some anti-cancer drugs are often absorbed by non-cancerous tissues and cells before they reach their intended target, which can lead to unwanted side effects and provide limited therapeutic benefit to the patient.
To address this issue, scientists have developed various methods to deliver drugs and nanomedicines to tumours. One approach is to use the patient’s own cells that cancer tissues tend to attract. These cells are extracted from the patient’s body, modified or infused with drugs in a sterile environment, and then reintroduced into the body to help destroy cancer cells via targeted drug delivery. However, this method is often costly, time-consuming, and carries the risk of cell contamination outside the body. In search of a better solution, Prof Wang’s team has developed a technique that enables nanomedicines to ‘hitch a ride’ on macrophages in the body. In this way, nanomedicines can reach cancer cells by taking advantage of the innate ability of macrophages, which are a type of immune cells, to migrate to and accumulate in areas of inflammation in the body, a process known as inflammatory tropism.
In essence, the team has developed a supramolecular nanomedicine that uses a macrocyclic compound called β-cyclodextrin (β-CD) as the host molecule and adamantane (Ada) as the guest molecule to modify, respectively, the surface of gold-nanoparticles (GNPs), which are used commonly in cancer therapy and diagnosis.
To create the supramolecular nanomedicine, the researchers coated the β-CD-modified GNPs and the Ada-modified GNPs with the membrane of E. coli outer membrane vesicles. This membrane serves as a protective barrier that prevents the GNPs from prematurely interacting with each other via β-CD-Ada host-guest interactions before they are administered to a patient. In addition, the E. coli membrane coating makes this nanomedicine ‘bacteria-mimetic’. Once intravenously injected into the patient’s body, the E. coli-mimetic nanoparticles are engulfed by macrophages.
The membranes of E. coli are then broken down by lysosomal enzymes, paving the way for supramolecular assemblies of β-CD-modified and Ada-modified GNPs into supramolecular aggregates. These aggregates are formed via supramolecular host-guest interactions, also personified as ‘hands-holding’. ‘The cancer tissue is the destination, the macrophages are the transportation vehicles, and the supramolecular medicines are the hitchhikers. Without the vehicles, the hitchhikers alone may not efficiently reach their intended destination,’ says Prof Wang.
Photothermal Cancer Therapy
Inside the macrophages, the supramolecular aggregates of GNPs also gain a photothermal property via ‘plasmonic effects’ as a result of the close contact of the gold surface of these nanoparticles. This means that they heat up when exposed to light, which doctors can use to their advantage. They can direct a specific laser wavelength to the site of the tumour once sufficient nanomedicines have gathered in them. This treatment, known as photothermal therapy (PTT), generates high temperatures that destroy the cancer tissue and cells.
The research team tested the efficacy of the bacteria-mimetic nanomedicine by performing PTT on mice with melanoma, a type of skin cancer. The mice were divided into three groups. The first group received gold nanoparticles without E. coli outer membrane vesicles, the second group received gold nanoparticles coated with E. coli outer membrane vesicles, and the third group received a combination of GNPs coated with an E. coli membrane vesicle, with half of the GNPs modified with host molecules and the other half modified with guest molecules.
After injection of these formulations, the first group of mice showed minimal accumulation of GNPs in the tumour, the second group exhibited improved accumulation due to the macrophage-hitchhiking driven by bacteria-mimics, while the third group showed a significant amount of GNPs deposited in the tumour, attributed to the intracellular supramolecular aggregation of GNPs that minimised premature loss during macrophage-hitchhiking delivery. When irradiated with laser, the solid tumour tissue in the third group of mice reached a very high temperature, yet no visible damage was found in the surrounding tissues apart from the tumour. This again indicates that most of the supramolecular nanomedicine had reached its intended target, the tumour tissue. Conversely, the tumour sites of the two other groups of mice showed a very modest temperature increase. Accordingly, when combined with anti-PD-L1, an immune checkpoint inhibitor, the bacteria-mimetic supramolecular nanomedicine exhibited the best therapeutic effect against melanoma, by having nearly eliminated all tumours from mice.
Towards Validation and Development
In 2022, the leading journal Science Advances published the team’s paper, titled ‘In vivo Hitchhiking of Immune Cells by Intracellular Self-assembly of Bacteria-mimetic Nanomedicine for Targeted Therapy of Melanoma.’ Prof Wang is the lead corresponding author and Simon Lee, distinguished professor in the ICMS, is the co-corresponding author. Postdoctoral fellow Gao Cheng and master’s student Wang Qingfu are the co-first authors. UM PhD students Li Junyan and Cheryl Kuong, research assistant Wei Jianwen, and postdoctoral fellow Xie Beibei also made important contributions to this research project. The project was funded by the Dr Stanley Ho Medical Development Foundation, the Macao Special Administrative Region Science and Technology Development Fund, the National Natural Science Foundation of China, and the Ministry of Education Frontiers Science Center for Precision Oncology at UM.
The research project was showcased in the 2022 Guangdong-Hong Kong-Macao Greater Bay Area High-value Patent Portfolio Layout Competition and received a silver medal, as one of only 25 gold and silver awardees, surpassing thousands of other entries. This success has drawn the attention of several companies in mainland China, which have expressed interest in partnering with the team to further develop and test the efficacy and safety of this innovative method.
Supramolecular Technology: A Versatile Tool to Improve Disease Therapies
Prof Wang’s team has conducted cutting-edge studies on the application of supramolecular technology for smart drug delivery in recent years, and this new method of cancer drug delivery is just one example. Another focus of the team is to combat acute pneumonia by enhancing drug delivery to the inflammatory lungs. To achieve this, the team has developed a technique that enables drugs to hitch a ride on red blood cells through a supramolecular interaction between β-cyclodextrin anchored on the cells, and ferrocene anchored on the surface of nanomedicine. ‘Red blood cells are biocompatible and have a long circulation time in the body, which makes them ideal carriers for drugs that need sustainable delivery.’ says Prof Wang. The team has found that curcumin, when delivered in this way, provides an effective treatment for acute pneumonia in mice.
Supramolecular technology also has the potential to control bleeding both inside and outside the body, which is a major threat to human life during surgery or after trauma. One way to tackle this issue is to inject plasma containing a lot of platelets, but they may not always reach the site of bleeding quickly. To enhance the platelets’ ability to target and clot injured blood vessels, Prof Wang’s team has used supramolecular interactions between cucurbit[7]uril (CB[7]), a type of macrocyclic molecule, and other compounds to modify platelets, turning them into ‘supramolecularly functionalised platelets’. In experiments with mice, the supramolecularly engineered platelets were found to greatly reduce the time and volume of bleeding compared to using normal platelets.
Supramolecular chemistry is a fascinating field that studies non-covalent bonding between molecules, from electrostatic interactions to hydrophobic effects. Research results in the field have already been translated into many everyday products and have great potential for the future. ‘Continued research into supramolecular systems will enable the design of increasingly smart structures that serve even more biomedical applications,’ says Prof Wang. ‘Tiny supramolecules have the potential to solve big problems for humanity, not just through drug delivery, but by unlocking an endless stream of ideas and solutions.’
Source: UMagazine ISSUE 27

Prof Wang Ruibing (centre) and his PhD students
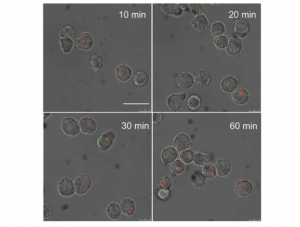
This figure shows the changes in RAW264.7 cells in mice at 10, 20, 30 and 60 minutes after they have been exposed to a mixture formed by two types of modified gold nanoparticles.
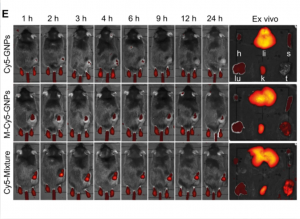
This image shows three groups of mice treated with gold nanoparticles at different times after injection
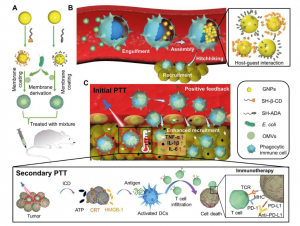
This figure shows how a supramolecular drug can hitchhike and self-assemble in immune cells inside the body

Prof Wang Ruibing’s research team was awarded a silver medal at the 2022 Guangdong-Hong Kong-Macao Greater Bay Area High-value Patent Portfolio Layout Competition

Prof. Ruibing Wang and his research group

