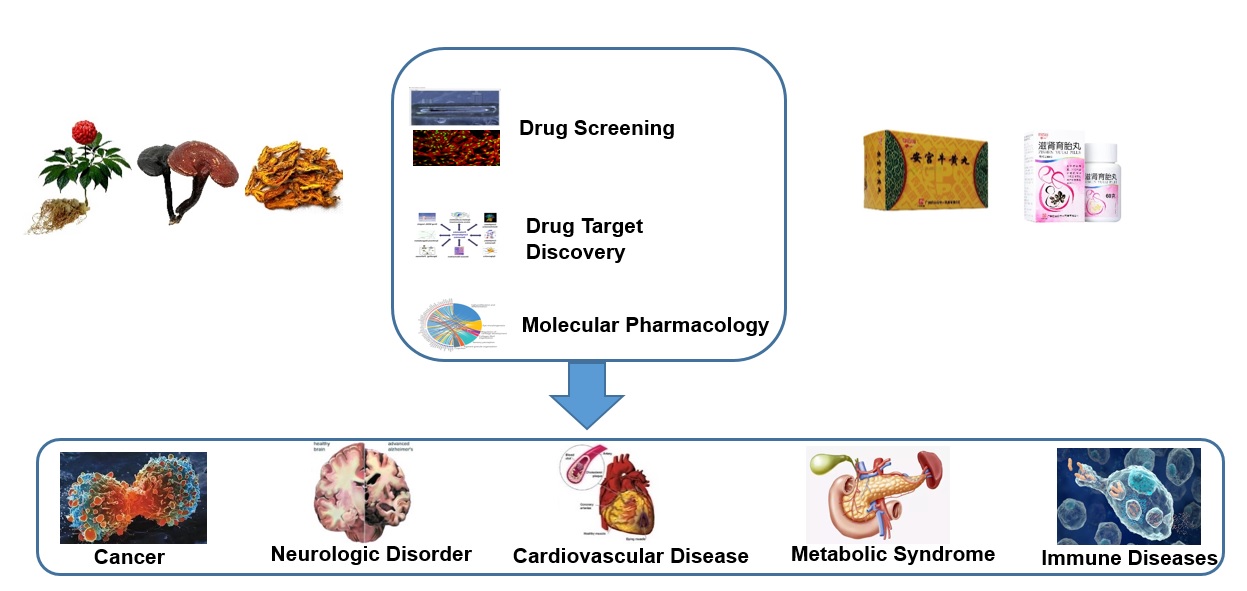
With the screening and evaluation of the efficacy of Chinese medicines as the core and Chinese medicines that are heat-clearing, body-strengthening, blood-promoting and both medicinal and edible as the research bases, we will focus on tumors, cardiovascular and cerebrovascular diseases, neurological diseases and metabolic diseases. We will establish screening platform based on molecules, cells, and zebrafish to screen Chinese medicines with active monomeric components or groups of active components that can potentially prevent and treat these diseases. We will also use techniques such as systems biology and molecular biology to explain the principle of action of Chinese medicines in preventing and treating these related diseases.
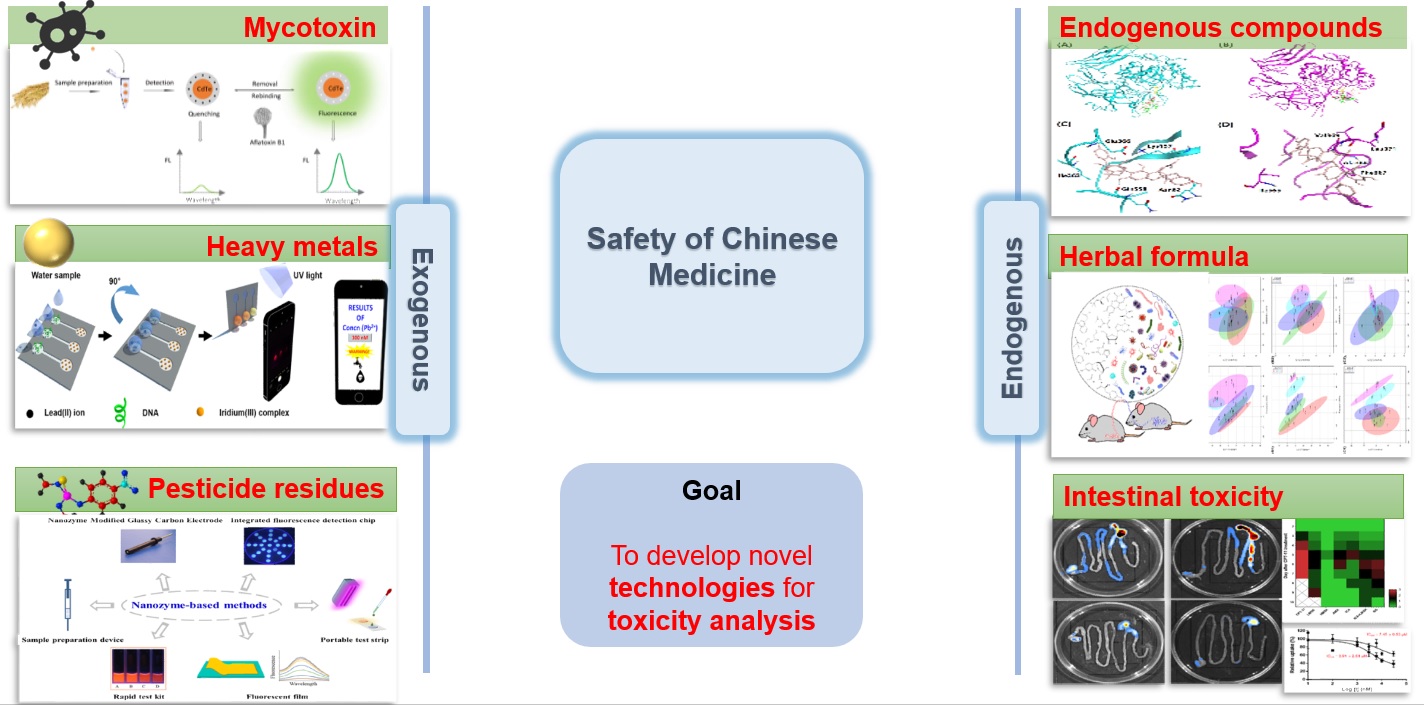
The safety of Chinese medicines is a major scientific issue concerning the modernization and internationalization of Chinese Medicine. The bottlenecks that restrict the development of the Chinese medicine industry include complex toxic components, unclear mechanisms of toxicity, and the lack of comprehensive and systematic rapid detection technology platforms for harmful residues. In response to the urgent needs and technical difficulties of safety evaluation, we will strengthen fundamental research on the safety of Chinese medicines from two aspects: toxicity evaluation and limit standards of endogenous toxic components and rapid detection technology for exogenous harmful residues.
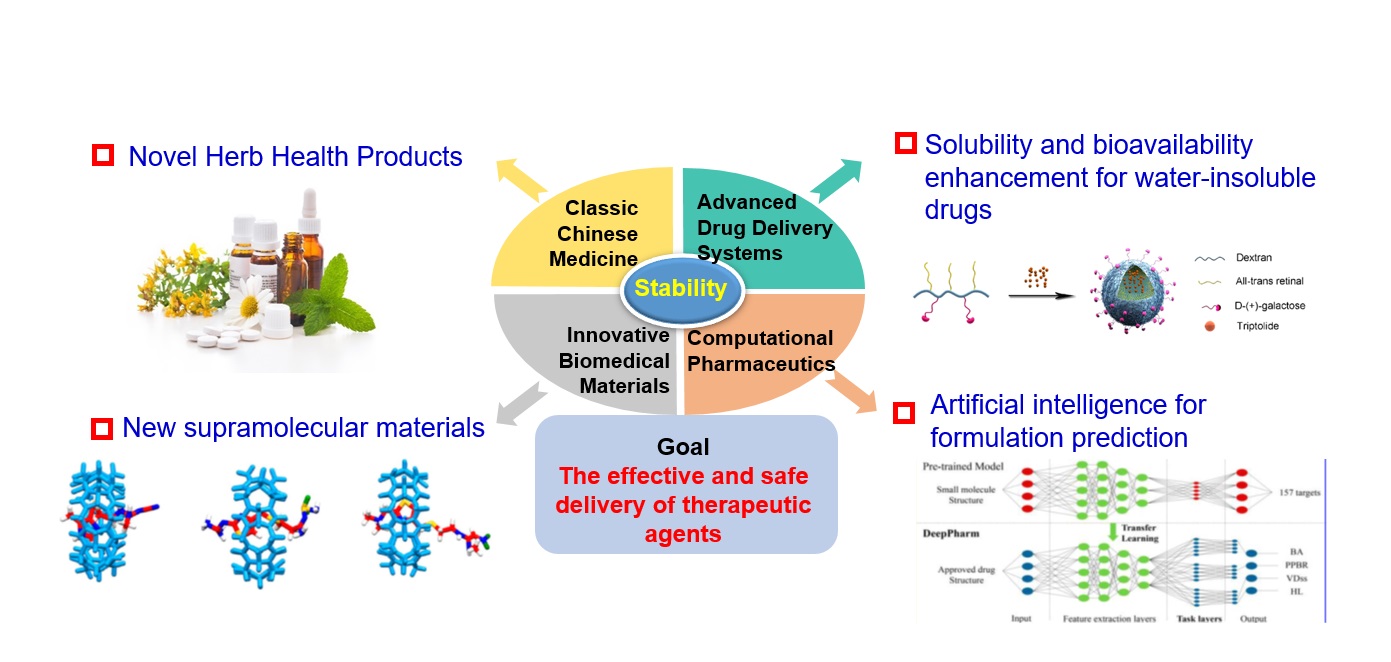
By integrating important theories and key technologies in multiple disciplines, we will explore the core issues of research on Chinese medicine preparations, including establishing new excipients and delivery systems for insoluble drug; and conduct research on the industrialization of multi-type and multi-source molecules of Chinese medicine as novel biomedical materials. We will also establish novel anti-cancer delivery system of Chinese medicine and strengthen research on its biological effects and mechanisms of action. On the other hand, we will develop computational pharmacy and apply multi-scale simulation, artificial intelligence and big data technology to the research of Chinese medicine preparations.
In view of the three main issues concerning the establishment of international Chinese medicine standards (the preparation of chemical reference materials for Chinese medicine, the selection of quality control indicators for Chinese medicine, and the key technology of quality control), we will begin with commonly used Chinese medicines and their main effective ingredients or components and make full use of modern advanced technology and methods to solve the joint application and optimisation of key technologies, establishing a reasonable, practical and effective quality control technology system.

