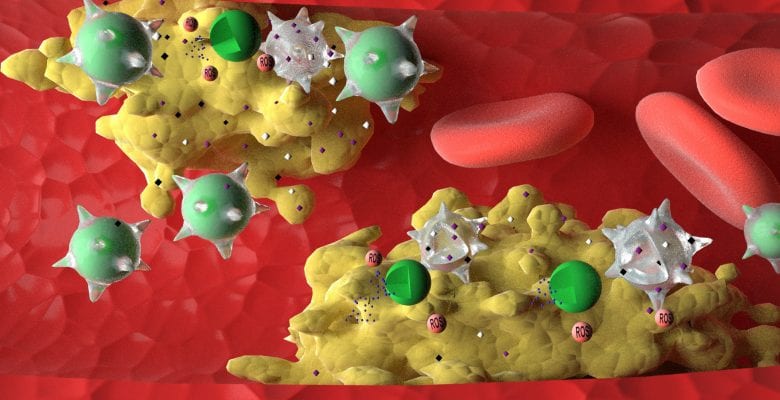
Nature Communications today (26 May) published a study from the University of Macau (UM) on the development of a biomimetic drug delivery system derived from macrophage-membrane coated reactive oxygen species (ROS)-responsive nanoparticles, which can effectively treat atherosclerosis without causing toxic side effects. This is also the first study in the field that compares a biomimetic nanomedicine with a live-cell-based drug delivery system in an inflammatory disease model, which can provide guidance for researchers to choose the appropriate formulation in future research.
Atherosclerosis is the main cause of cardiovascular and cerebrovascular diseases, such as coronary heart disease and cerebral infarction, which seriously threatens human life and health. It is the result of atherosclerotic plaques depositing on the walls of blood vessels, which causes the narrowing of arteries. Although existing drugs can slow the progress of the disease, they cannot cure the disease and tend to cause toxic side effects. To address this problem, a research team led by Prof Wang Ruibing and another team led by Prof Li Ming Yuen at UM’s Institute of Chinese Medical Sciences worked together and developed a biomimetic nanomedicine derived from ROS-responsive nanoparticles coated with macrophage membrane, which can effectively reverse the atherosclerosis process in mice without causing toxic side effects. The study is also the first of its kind in the field to compare the efficacy of the new biomimetic nanomedicine with that of emerging live-cell based drug delivery system, with detailed explanation of the reasons behind their differences.
Based on the overproduction of ROS in atherosclerotic plaques, the research team designed and prepared ROS-responsive nanoparticles loaded with atorvastatin as a model drug. They further developed a macrophage-biomimetic nanomedicine by coating ROS-responsive nanoparticles with macrophage membranes. They also prepared a ‘live-cell’ drug delivery system by internalising ROS-responsive nanoparticles inside macrophages. During the treatment of atherosclerosis in mice, macrophage-biomimetic nanomedicine efficiently accumulated in the atherosclerotic plaques, released atorvastatin in response to the locally overproduced ROS, and effectively ameliorated and reversed the atherosclerotic plaques formation, with better therapeutic efficacy than the live-cell drug delivery strategy.
‘This is mainly because macrophage membranes in our biomimetic nanomedicine are able to efficiently sequester multiple proinflammatory factors at the plaque sites, thereby reducing the level of inflammation. The macrophage membranes work synergistically with atorvastatin to exhibit significant anti-inflammatory effects during the treatment of atherosclerosis,’ says Prof Wang. ‘In contrast, when used as a drug delivery vehicle, the live macrophage, in spite of its targeted accumulation in the plaques, may get activated by local inflammatory factors, leading to elevated inflammation. Therefore, the live-cell drug delivery system in our study was not as effective as the biomimetic nanomedicine for the treatment of atherosclerosis.’
This research study was supported by the Science and Technology Development Fund, Macau SAR (File No. 0121/2018 / A3) and UM’s research fund. Associate Professor Wang Ruibing and Professor Lee Ming-Yuen are the corresponding authors of the article. PhD students Gao Cheng and Huang Qiaoxian (UM Macao PhD Scholarship recipient), as well as master’s student Liu Conghui are the co-first-authors of the article. Associate Professor Wan Jianbo, PhD student Yue Ludan, and master’s student Kwong Cheryl H. T. also made important contributions to this study.
Titled ‘Treatment of Atherosclerosis by Macrophage-Biomimetic Nanoparticles via Targeted Pharmacotherapy and Sequestration of Proinflammatory Cytokines’, the paper has been published by Nature Communications, a leading comprehensive journal under the world-renowned Nature. For more information about the study, please visit: https://www.nature.com/articles/s41467-020-16439-7
Source: https://www.um.edu.mo/news-centre/news-and-events/news-and-press-releases/detail/50192/