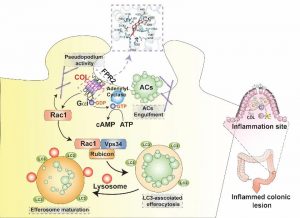
A joint research collaboration between the University of Macau and the Chinese University of Hong Kong (Shenzhen) had made important breakthrough in the field of traditional Chinese medicine small molecule for the treatment of inflammatory bowel disease (IBD). The study successfully identified a natural small molecule from traditional Chinese medicine that activates the efferocytosis activity of macrophages. This small molecule selectively activates the formyl peptide receptor-2 (FPR2) on the surface of macrophages, enhances efferocytosis activity through non-classical autophagy, and promotes tissue repair in a mouse model of inflammatory bowel disease. This research provided new insights and strategies for the treatment of IBD and reveals the unique pharmacological mechanism of Chinese medicine in anti-inflammatory. The relevant research had recently been published in the internationally renowned journal EMBO Molecular Medicine.
The effective clearance of apoptotic cells, which arise in large numbers in adults every day, is crucial for maintaining organism homeostasis. Efferocytosis is an important mechanism for the body to clear aging and dying cells, and it contributes to embryonic development, organ formation, tissue repair, and immune regulation. Previous studies by the research team led by Dr. Jiahong Lu suggested that impaired efferocytosis is involved in the process of inflammatory bowel disease, and enhancing efferocytic activity may be an effective strategy for the treatment of IBD. However, the current research on drug targets that regulate efferocytic activity is relatively weak, and there is a lack of small molecules that can effectively enhance efferocytic activity for pharmacological evaluation. This study identified a small molecule called Columbamine derived from Coptidis Rhizoma, which can effectively enhance the efferocytic activity of macrophages and exhibit significant anti-IBD effects. To further elucidate the regulatory mechanism of this small molecule, the team combined clinical data analysis, transcriptomics analysis, cell and molecular biology experiments, and gene knockout animal validation. They found that the FPR2 is the drug target through which Columbamine regulates macrophage efferocytic function. FPR2 is mainly expressed on the surface of innate immune cells and is a G protein-coupled receptor involved in immune regulation. Depending on its different regulatory patterns, FPR2 can exert different immune regulatory functions. It was verified that Columbamine can directly bind to FPR2, promote cAMP response, activate LC3-related phagocytic capacity, and ultimately enhance efferocytic activity, alleviate the damage of inflammatory bowel disease in mice, and promote tissue repair.
The corresponding authors of this study are Dr. Jiahong Lu, Associate Professor at the Institute of Chinese Medical Sciences, University of Macau, and Professor Dequan Ye, leading a team at the School of Life and Health Sciences, Chinese University of Hong Kong (Shenzhen). The co-first authors are Dr. Mingyue Wu, Postdoctoral Researcher at the University of Macau (currently Associate Researcher at the Department of Gastroenterology, Southwest Hospital, Army Medical University), Dr. Yunjun Ge, Lecturer at the School of Medicine, Jiangnan University, and Erjin Wang, Ph.D. student at the University of Macau. The Biological Imaging & Stem Cell Core and Animal Research Core at the Faculty of Health Sciences provided strong support for the research. This research was supported by the Shenzhen Fund for Basic Research (SGDX20210823103804030), the Science and Technology Development Fund of Macau S.A.R. (0025/2022/A1, 0128/2019/A3), the University of Macau Research Fund (No. MYRG2022-00094-ICMS), the National Natural Science Foundation of China (82271455; 32070950; 82104183, 82200585), the Natural Science Foundation of Guangdong Province (2022A1515012416), the Special Fund for Guangdong-Hong Kong-Macao Joint Laboratory (2020B1212030006), and the Shenzhen Municipal Science and Technology Innovation Committee (GXWD20201231105722002-20200831175432002, JCYJ20200109150019113).
Link to the original article: https://www.embopress.org/doi/full/10.15252/emmm.202317815