https://mp.weixin.qq.com/s/4V8XLqes06SWemXqg59T8g
A research team led by Yuanjia Hu, the professor of State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, and Department of Public Health and Medicinal Administration, Faculty of Health Sciences at the University of Macau, with the team of Herui Yao, the professor of Sun Yat-sen Memorial Hospital of Sun Yat-sen University, have made a significant breakthrough in the analysis of the global patent pattern of HER2 biologics. The research team conducted a summary statistics on the current HER2 biologics patents, and included the biosequence analysis of HER2 monoclonal antibodies (mAbs) for the first time. Relevant research results have been published in the international academic journal Nature Biotechnology.
Human epidermal growth factor receptor-2 (HER2) is a receptor tyrosine kinase of the epidermal growth factor receptor (EGFR) family. Mutations of HER2 are closely related to the occurrence and development of tumors. Over the past 20 years, a variety of drugs or drug candidates targeting HER2 have emerged in market. Patents are critical to developers and stakeholders as they protect innovation interests and commercial value. Meanwhile, the patent data itself also has important research value.
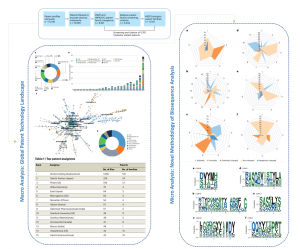
The research team summarized the current patents of HER2 biologics. The field of HER2 biologics is dominated by Roche with 1092 patent applications, accounting for 22% of all patent applications. From 50 patent families in 2018 to 288 in 2021, patent applications for HER2 biologics showed a rapid growth trend. At the same time, relatively new technologies, such as ADCs (antibody drug complexes) and cell therapy, also developed rapidly.
It is worth noting that this study is the first to analyze the biological sequence of HER2 mAbs. In the amino acid sequence composition, the canonical structure of CDR usually plays an important role in maintaining the conformation of the CDR region, while sequence diversity is critical to the antibody-antigen recognition mode. The 20 amino acids were divided into five categories: aliphatic, aromatic, positively charged, negatively charged and uncharged amino acids. Key antibody complementarity-determining region (CDR) sequences, including heavy and light chains, were screened and further analyzed from the core patent families. The study summarized the classification patterns of amino acids in related CDR sequences, as well as conserved and hypervariable regions in CDR sequences. Among the 1,003 HER2 biologics patent families, 423 families contained biosequences, and 47 mAb patent families were selected for further analysis. About 36% of the mAb families focused on the optimization and improvement of existing antibodies, such as the optimization of trastuzumab or pertuzumab. Approximately 64% of the patented families belonged to novel HER2 mAbs with different binding domains compared to existing antibodies. HER2 mAb patents reflect the current status and trends of research and development in this field, including but not limited to improving drug resistance, reducing immunogenicity, enhancing antibody-dependent cellular cytotoxicity (ADCC), enhancing target selectivity and improving formulation stability. The aim of the biosequence analysis on HER2 mAbs was to explore new forms of patented biological sequence research, and provide a methodological reference for antibody modeling and rational design, as well as antibody modification and optimization. In conclusion, HER2 biologics were booming from the patent perspective. Besides emerging mAb and ADC drugs, multispecific antibodies, cell therapy, and various novel biologics are new trends in HER2-targeted therapy.
Professor Yuanjia Hu from the University of Macau and professor Herui Yao from the Sun Yat-sen University are the co-corresponding authors of the article, while Dr. Qingjian Li from the Sun Yat-sen University, Jiaqi Xu PhD student from the University of Macau, and Qianshu Sun master graduate from the University of Macau, are the co-first authors. Zebang Zhang PhD student from the Sun Yat-sen University has also contributed to the study. The project is supported by the National Science and Technology Major project (2020ZX09201021), the National Natural Science Foundation of China project (8197247, 82273204 and 82203086), the Sun Yat-sen University Clinical Research 5010 Program (2018007), the Guangzhou Science and Technology Major Program (202206010078), the Guangzhou Science and Technology Program (202201020574 and 202201020246), the Medical Artificial Intelligence Project of Sun Yat-sen Memorial Hospital (YXRGZN201902), the CSCO-Hengrui Cancer Research Foundation (Y-HR2018-284), the Guangdong Basic and Applied Basic Research Foundation project (2020A1515110756 and 2023A1515010916), the China Postdoctoral Science Foundation (2020TQ0377 and 2020M683168), the Zhuhai UM Science and Technology Research Institute (ZUMRI/064/2021) and the Science and Technology Development Fund of the Macao Special Administrative Region (SKL QRCM(UM)-2023-2025). We gratefully acknowledge the support of the Derwent Innovation (DI) patent database of the University of Macau Library. The full version of the research article can be viewed at: https://doi.org/10.1038/s41587-023-01814-8.