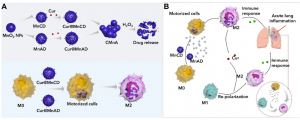
A research team led by Wang Ruibing, associate professor in the Institute of Chinese Medical Sciences (ICMS) at the University of Macau, has developed a supramolecularly engineered motorised cell platform as a nanomedicine carrier, based on the excellent inflammatory tropism of macrophages and the catalytic generation of oxygen as the propelling force. When loaded with anti-inflammatory curcumin, the platform showed a good therapeutic effect against acute pneumonia. The research results have been published in the top academic journal Advanced Materials.
Curcumin, as a widely studied small molecule isolated from Chinese medicinal herb Turmeric, has shown good therapeutic potential in the treatment of many inflammatory diseases. However, curcumin itself does not target inflammatory tissues. That is, when curcumin is used alone, it cannot accurately accumulate in the inflammatory tissues, thus the clinical results have not been satisfactory. In recent years, living cells have received increasing attention as drug carriers because they are highly biocompatible and are less likely to cause adverse reactions. Moreover, living cells have the characteristics of extended circulation. They can prolong the drug residence time in the body and transport drugs to a specific tissue, thereby increasing the local concentration and efficacy of the drug in that tissue. However, the delivery of small-molecule drugs such as curcumin by living cells may result in intracellular metabolism or efflux during during delivery.
The research team loaded curcumin molecules into manganese dioxide nanoparticles modified by host and guest molecules respectively, and subsequently incubated macrophages with these nanoparticles sequentially to induce intracellular supramolecular self-assembly, in order to lock the drug-carrying nanoparticles in the macrophages to inhibit efflux. Due to their inflammatory tropism, the drug-loaded macrophages accumulated in the inflammatory lung tissues. In the highly oxidative microenvironment at the site of inflammation, the manganese dioxide nanoparticles catalysed hydrogen peroxide to generate oxygen, driving the macrophage robot to quickly infiltrate the inflammatory tissue. Meanwhile, curcumin was released in the inflammatory tissue, polarising macrophages, and jointly regulating the inflammatory microenvironment, to reverse inflammation.
Previously, based on supramolecular self-assembly technology, the research team developed a series of living-cell-based drug delivery platforms, and the research results were published in top journals such as Nature Communications, Science Advances, Materials Today, and Advanced Functional Materials. The team’s unique supramolecular cell engineering technology relies on the strong host-guest interactions to lock nanoparticles in cells or anchor them on the cell surface, in order to realise targeted enrichment and the precise release of drugs using living cells. This technology effectively regulates and treats inflammatory diseases with high specificity, and the team received a second prize in the Natural Science Award category at the Macao Science and Technology Awards 2022 for this achievement.
Building on their previous works, members of the research team designed this self-propelled cell robot that can rapidly infiltrate inflammatory tissues, facilitating rapid intervention in the development of acute inflammation. The study titled ‘Chemotaxis-guided Self-propelled Macrophage Motor for Targeted Treatment of Acute Pneumonia’ has been published in the top academic journal Advanced Materials. Prof Wang is the sole corresponding author of the paper and UM PhD student Yue Ludan is the first author. In addition, ICMS Distinguished Professor Lee Ming Yuen, Research Assistant Professor Gao Cheng, and PhD students Li Junyan, Chen Hanbin, and Luo Ruifeng also made significant contributions to the study. This study was funded by the Science and Technology Development Fund of Macao SAR (File no: 0086/2022/A2 and 0065/2021/A2), the National Natural Science Foundation of China (File no: 22271323 and 22071275), and the Shenzhen Science and Technology Innovation Commission (File no: EF023/ICMS-WRB/2022/SZSTIC).
To view the full version of the paper, please visit: https://onlinelibrary.wiley.com/doi/10.1002/adma.202211626.