- Admission is open to applicants who have completed at least a 4-year Bachelor’s degree or equivalent in related fields from an accredited institution
- Please visit our Graduate School website to find out more about admission and application procedure: https://www.um.edu.mo/grs/en/admissions_pro_info.php
Applicants who received their Bachelor’s degree from a university where the medium of instruction was not English are required to provide any of the following as proof of English proficiency:
- Obtain a TOEFL score* of 550 (paper-based examination) / 80 (internet-based examination) or,
- Obtain an IELTS overall score* of 6.0 with no sub-score lower than 5.5 or,
- Obtain a CET-6 score of 430 or above or,
- Pass the TEM-4 or TEM-8 examination
*Note: TOEFL and IELTS scores are valid for two years from the test date.
Applicants who received their Bachelor’s degree from a university where the medium of instruction was English are required to provide a proof of English as the medium of instruction, if deemed necessary, issued by the applicants’ university where their Bachelor’s degree was obtained.
Individuals involved in medicinal administration help create an environment in which the health of a population or patient groups is protected and promoted both effectively and efficiently. They are the professionals in a dynamic and interdisciplinary field who may have tremendous influence on the availability, accessibility, and quality of pharmaceutical and related products, drawing on a balance between the business side of pharmaceutical development and providing top quality and highly effective treatment options. The practicality of their expertise may lie anywhere in the lifecycle of pharmaceutical products encompassing research and development, patent approval, marketing authorization, enlistment and procurement, supply and distribution, rational use, pharmacovigilance, etc.
Featured with a high degree of applicability and practicality, this programme aims to address the need for high-end talents in the medicinal sector who can skillfully use the theories, methods and techniques of modern sociology, economics, law and regulations, project planning and management, and/or statistics to resolve real-life issues in the practical setting of pharmaceutical industry, innovation and technology, and other aspects of medicinal administration. In alignment with the prime academic goal of the Institute of Chinese Medical Sciences (ICMS) and State Key Laboratory of Quality Research in Chinese Medicine (SKL-QRCM), this programme supports the development of professionals in medicinal administration for Macao and beyond.
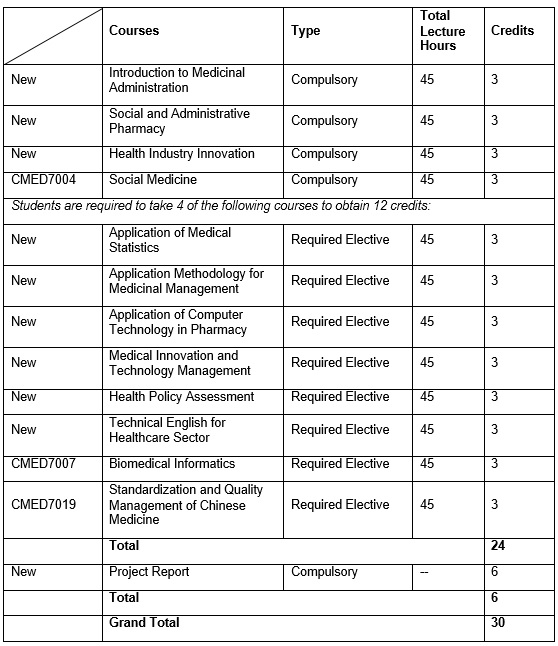
In the 1st Academic Year, students are required to complete 4 Compulsory Courses and any 4 Elective Courses (chosen from a list of Elective Courses). In the 2nd Academic Year, students are required to complete a Project Report before graduation.
- Pharmaceutical companies (management departments, such as finance, personnel, sales, marketing): The graduates shall be able to perform specific tasks of overseeing drug development from start to finish, and be able to identify and resolve any technical requirements related to the pharmaceutical affairs within budget and in line with all scientific requirements for safety and efficacy. They will also acquire the skills and knowledge to manage pharmaceutical projects, stay up to date on all applicable regulatory guidelines, and assess the quality, safety and efficacy of pharmaceutical products.
- Health policy analysts: The graduates shall work for educational institutions, research labs, hospitals, non-profit organizations and different types of community agencies with a specific focus on researching about, making reports of and managing health care policies and programs. They will acquire skills and knowledge in collecting and analyzing statistical data for health policy development or health programme design and implementation.
- Health services managers at hospitals, community health organizations, clinics, and other healthcare organizations: The graduates will acquire the skills and knowledge in managing medical and health services overseeing the overall operations of their place of work and ensuring the quality of care to patients. They will be equipped with management skills in managing staff, maintaining budgets, coordinating delivery of care with their workplace and across different sectors, and developing and implementing policies to enhance the quality of case and the efficiency of the healthcare institutes.
- Self-employment: medical market and product research and development information agency, venture capital company consulting department.
Besides the courses and research projects, ICMS provides our students a resourceful communication platform and an enjoyable extracurricular life. Students will have the opportunities to attend conferences and join exchange activities all over the world. The institute also schedules regular visits to our partners in the pharmaceutical industry for our students Starting from a warm welcome to the freshmen, the ICMS Postgraduate Association organises various activities throughout the year, e.g. Mid-Autumn Festival celebration, photographic competition, academic forum and so on.
OUR EXCHANGE PARTNERS
- Peking University
- China Pharmaceutical University
ESSCA of Hungary
Tuition Fees: http://www.um.edu.mo/grs/en/tuition_fees.php
Assistantship: https://www.um.edu.mo/grs/en/admissions_master_assistantship.php
Postgraduate House (PGH) consists of 4 buildings (S1, S2, S3 and S4), offering more than 2,600 bed spaces mainly to UM non-local students. All the rooms in the PGH are fully furnished, internet-connected, air-conditioned and with bathrooms, excluding personal necessities and beddings. Please visit the following links for more information:
Postgraduate House:
https://www.um.edu.mo/sao/srs/sh/aboutus/en/srs_management.php
Fees and Charges:
https://www.um.edu.mo/sao/srs/sh/en/srs_fees.php
For more details, please visit our Registry and Graduate School websites:
Registry –https://reg.um.edu.mo/
Graduate School –http://www.um.edu.mo/grs/en/
- Understand, explain, integrate, and articulate the information derived from social-medical interactions across the disciplines of social and administrative pharmacy, health industry innovation and social medicine to address the issues in medicinal administration.
- Identify and appraise emerging theories and recent development of interventions, policies, evidence, and technology innovation in medicinal administration.
- Apply the knowledge about pharmacy, medicine, sociology, innovation and technology, economics, policies/law/regulations, project planning and management, and/or statistics for problem-solving in the practical setting of medicinal administration.
- Develop, communicate about and execute pragmatic solutions that employs a problem-orientated approach to resolve real-world issues or to promote innovation in medical administration.
(Remarks: The Programme Intended Learning Outcomes (PILOs) to be launched in AY2024/25 and programme registration is still in progress.)

